Specialized Information for:
Long-Term Care ConsumersFamily MembersAdvocates
Specialized Information for:
Long-Term Care ConsumersFamily MembersAdvocates
April 12, 2023
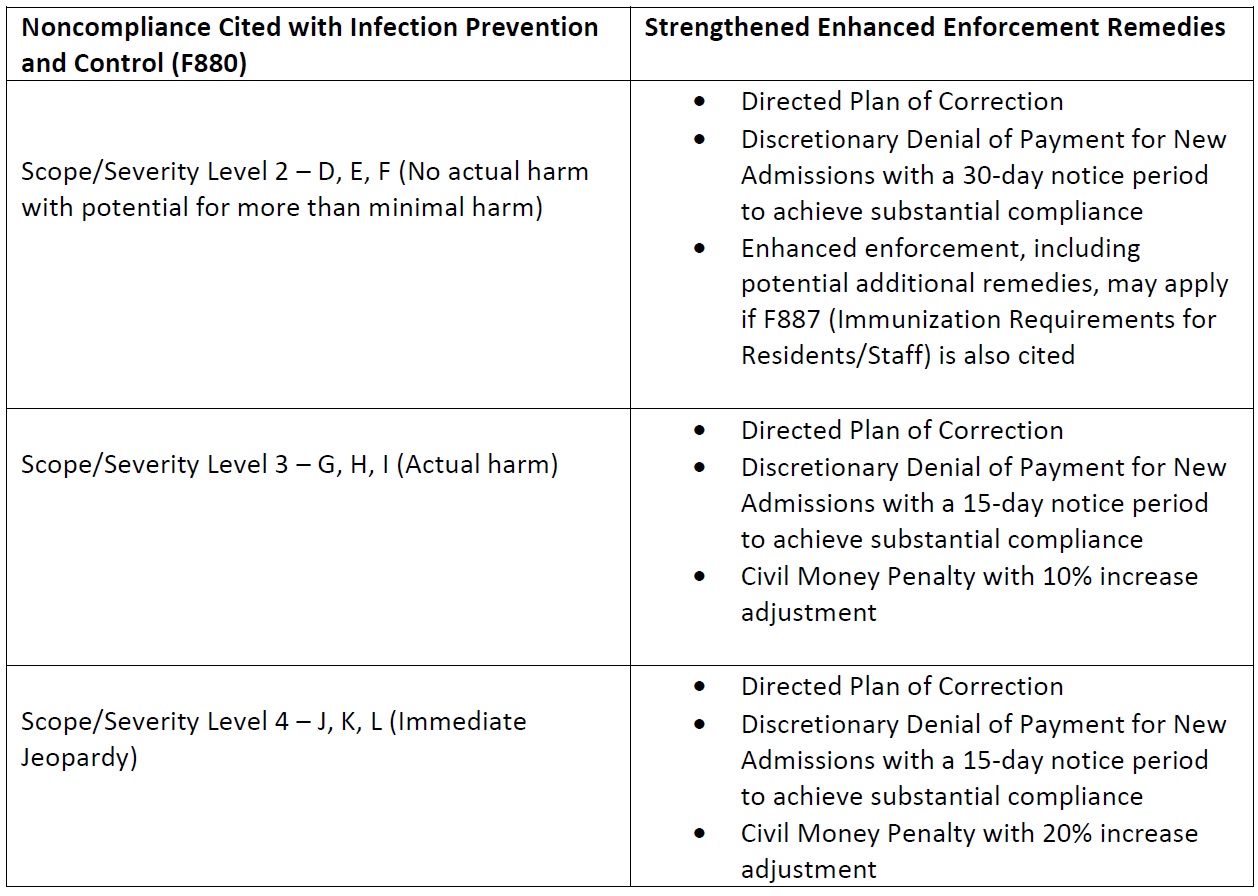
On March 30, 2023, the Centers for Medicare & Medicaid Services (CMS) issued revised guidance, QSO-23-10-NH, “Strengthened Enhanced Enforcement for Infection Control Deficiencies and Quality Improvement Activities in Nursing homes,” which strengthens enforcement efforts for noncompliance with infection control deficiencies (F880) and vaccine immunization requirements for staff and residents (F887).
Consumer Voice has long been advocating with CMS and the Administration for stronger enforcement measures. Prior to the COVID-19 pandemic, noncompliance with Infection Prevention and Control was the top deficiency cited. Despite the devastation nursing home residents faced throughout the pandemic, most violations inspectors found were labelled as causing “no actual harm,” which means facilities rarely faced any meaningful financial penalties. The revised guidance directly responds to Consumer Voice’s concerns over lack of enforcement, targeting “facilities with, or at risk for, the most significant negative resident health outcomes.”
Among other things, facilities that are now cited with Actual Harm for non-compliance for Infection Prevention and Control (F880) will face a 10% increase in Civil Money Penalties with a ten percent increase adjustment; and facilities cited with Immediate Jeopardy will face a 20% increase in Civil Money Penalties.